Physician's Notebooks 2.- http://physiciansnotebook.blogspot.com - See Homepage
Student! Reading and re-reading this chapter may up your IQ, SAT, MCAT, TOEFL scores and help get you low- or no-cost college education.
2.9c: Protein & Amino Acids; (Update, 25 Nov.2021)
The below column has subjects in order of appearance in text. Use search & find or scroll to find in text.
(Note: the arrangements of atoms in some formulas here have got out of place. Also a few images have been lost on Internet. Still it is useful chapter; and use Wikipedia to supplement)
Proteins are the 3rd caloric major nutrientThe most important proteins are made of alpha-amino acids
The Amino Acid prototype glycine in proteins
The Alanine Group
The Alkaline and Acidic Derived Amino Acid Group
The Sulfur Amino Acids Group
The Cyclic AA that make the main Neurotransmitters —-phenylalaninePhenylketonuria
The 5-Sided NH Cyclic Amino Acids - Proline and Histididine
Schizophrenia and Hyperprolinemia
Amino Acids and Genetic-Code Proteins
The 5-Sided NH Cyclic Amino Acids - Proline and Histididine
Schizophrenia and Hyperprolinemia
Amino Acids and Genetic-Code Proteins
The Essential Amino Acids
Sources of Protein
Protein Digestion
Nitrogen Excretion – Creatinine, Urea and Ammonia
Sources of Protein
Protein Digestion
Nitrogen Excretion – Creatinine, Urea and Ammonia
Proteins are the 3rd caloric major nutrient (after carbohydrates and lipids) and they differ from the other two, structurally, by having the atom nitrogen, N, and by being more important for structure than for function. Proteins can be used to produce energy by oxidation but that is mostly a waste of good structure.
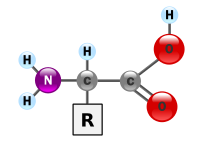
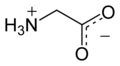
The most important proteins are made of alpha-amino acids: An alpha-amino acid is in the following structure: (Check the Internet for alpha amino acid)
(Left to right in the above schematic) H2N-CHR-COOH. It has, on its end to your left, the amino -NH2 and, on its end to your right, the carboxyl -COOH. The central structure, which you see in the Figure is the vertically clockwise rotated H-C-R and can be built up from the simplest R, in which the R = 0 (no) Carbon C and 1 (one) Hydrogen H, as in the simplest alpha-amino acid glycine,
H H OH
H2N-C–C=O
H H
into the more complex molecular structures as we shall see below in the various alpha-amino acids that make up proteins in living things. The "alpha-amino acid" refers to a type of amino acid where the attachment of the main amino, -NH2 is to the first Carbon (C opposite the -COOH attachment). From here, in this chapter, I may refer to alpha-amino acid simply as amino acid or AA.
In terms of its chemical element atoms, a protein has Nitrogen N (Uniquely), carbon C and Hydrogen H. Also Sulfur S and Phosphorus P are in some proteins. The carbohydrates and fats form structural components with proteins as glyco- and lipo-proteins. Combinations of the alpha-amino acids structurally define each protein encoded by the universal genetic code in our DNA in the 23 human chromosomes.
The Amino Acid prototype glycine in proteins is like the prototype glucose in carbohydrates and the prototype glycerol in lipids. It is based on the 1 central-carbon structure of Methane, the famous marsh gas.
In Methane
Methane
a central carbon C is bonded to four H-atoms at clockwise intervals, the 3, 6, 9 and 12 o'clock positions.
The Structure of glycine:.
In glycine you see
substituted for the 3 o'clock H atom of the methane, a –COOH carboxyl radical. At 9 o'clock on the central C atom, the NH2 amino radical is substituted for the 9 o'clock H in methane. (Usually denoted –NH2 but here showing the 2 H's of NH2 are each bonded separately, one on side and one on top, to the N, which bonds on its other side to the central C atom)The -COOH, carboxyl part of the AA molecule, has the property of being acidic because it easily loses the terminal H+ of its OH (Becomes COO–, liberating the H+ ion in solution, the definition of acid); while the NH2 part of the molecule has the property of being a base, or alkaline, because it attracts H+. Thus, amino acids are acid on one end of the molecule and base (alkaline) on the other end. This has an important result that 2 AA can react, as an acid will react with a base, producing a union of the reacting AAs, called peptide bonding. This is the start of protein synthesis: one AA will react with another to form a dipeptide; then another AA tacks on to an end of the dipeptide to form a tripeptide; and so on until a molecular structure of thousands of AA, called polypeptide, is formed; and when the synthesis is completed, the large polypeptide fits the definition of protein.
The Amino Acid Structures
Glycine’s relation to the structure of methane is a key for student's memorizing all the AA structures: firstly the Alanine Group. From glycine, the other groups of alpha-amino acids start with the Alanine Group, which are easily seen to be substitutions for the middle -CH3 Hydrogen H atoms as inAlaninethe
Alaninenot need to be present in the diet.
(Note in Serine in the methyl radical a CH2OH replaces the CH3)Valine is Alanine with its CH3 having two of the H atoms replaced (the CH3 becomes CH) each by a –CH3.Leucine is Valine with a CH2 inserted between the central C and the CH of Valine.Isoleucine is Leucine with one of its lower –CH3 having an H atom replaced (and becoming CH2) by an added CH3.HSerineH2N-C-COOHCH2OH
Then consider the alkaline and acidic derived amino acid group so called because they have extra alkaline (-NH2) or acidic (-COOH) insertions. And included with them are same-initial letter de-alkalinized or de-acidified twins, e.g., Asparagine with Aspartic Acid and Glutamine with Glutamic Acid,Threonine is the ethyl alcohol analog of serine.In Threonine the –CH2OH methyl alcohol radical is replaced by the –CH2CH2OH ethyl alcohol radical.
The Alkaline and Acidic Derived Amino Acid GroupAsparagine (your left) & Aspartic Acid (right)H HH2N–C–COOH H2N–C–COOHCH2 CH2C=O C=ONH2 O-H+In Asparagine: two NH2 and one COOH unbalance the molecule in alkaline direction. Aspartic Acid is asparagine with acidic –O-H+ replacing alkaline lowest NH2 creating a 2nd –COOH and an acid imbalance. And note both are structured from the methane/glycine/alanine central C matrix.
Glutamine (your left) and Glutamic Acid (on right)H HH2N–C–COOH H2N–C–COOHCH2 CH2CH2 CH2C=O C=ONH2 O-H+Glutamine is asparagine with an extra CH2 as above. Similarly glutamic and aspartic acids. Glutamic acid (glutamate) is a major excitatory neurotransmitter in the human brain, and also the immediate source of gamma amino butyric acid (GABA) the major inhibitory neurotransmitter. A big source of glutamate in the diet is soy sauce. We should not forget when we eat soy sauce that too much of it can cause excessive stimulation of the psyche.
Lysine is like glutamine and arginine but with extra –CH2’s inserted. Lysine is strongly alkaline due to double NH2.In arginine, see glutamine with 3 instead of 2 CH2’s and one H atom of the lowest NH2 replaced by the H2N–C=NH radical at its C atom bonding point. The extra NH & NH2 make it strongly alkaline. Arginine is the source of the important gaseous neurotransmitter Nitric Oxide (NO), which causes the blood pressure to drop because it relaxes the small arteries and also is involved in Viagra stimulated erections.
ArginineHH2N–C–COOHCH2CH2CH2NHH2N–C=NH
LysineHH2N–C–COOHCH2CH2CH2CH2NH2
The Sulfur Amino Acid Group
CysteineHH2N- C—COOHCH2SHCysteine is alanine with an H of the CH3 replaced by –SH as shown. Two cysteine molecules joined by each one’s sulfur atom (S) in a so-called disulfide-link bond is the dipeptide, cystine. It is less soluble and forms kidney stones in special metabolic illnesses or acidic conditions of the body. The –S-S- bond is important in forming key proteins. Another important cysteine-linked food additive is glutathione
in which cysteine is linked to glutamate at the NH2 end as a tripeptide. It is an anti-oxidant that protects the brain against Alzheimer’s and Parkinson Diseases.MethionineHH2N- C—COOHCH2CH2SCH3Methionine resembles Cysteine but with insertion of the extra CH2 above the S atom. The vitamins folic acid and B12 are important for methionine because they assist enzymes in methylation reactions that attach the -CH3.Note that the sulfur amino acids make the yellow in egg yolk and also are high in meat. When metabolized, they liberate excess H+ and worsen the acidosis of renal failure. But they are very essential in nutrition.
The Cyclic AA that make the main Neurotransmitters—-phenylalanine, et. al. that follow here are important sources of the key neurotransmitters - dopamine, norepinephrine, epinephrine and serotonin. Anti-depression and anti-psychosis medications affect at least one of these neurotransmitters. The theories of clinical depression and schizophrenia involve them. One of the cyclic amino acids, Tryptophan, also is the source of the pineal gland sleep hormone, Melatonin.
Phenylalanine
Phenyalanine should be immediately seen to be derived by replacing an H of alanine's -CH3 with the cyclic 6-carbon phenyl fragment.
In the genetic autososomic recessive 1ine, the 10 to 20 thousand incidence mutation disease, Phenylketonuria, (PKU); the unconverted, blocked up Phenylalanine can become highly toxic to the brain and is recognized by testing the urine because the enzyme phenylalanine hydroxylase that gets rid of excess phenylalanine is defective. The treatment is a low-protein, phenylalanine-free diet from birth. Note that the dietary treatment of phenylketonuria must start at birth, be carried out by an expert team, and continued strictly throughout life. Otherwise some degree of mental retardation is inevitable. Many other similar metabolic protein enzyme mutation deficiencies afflict humans; PKU should be a model for dealing with them.
Tyrosine,

Note that phenylalanine can form tyrosine by attaching a bottom -OH. Then tyrosine goes through conversions
(See below the schematic between Tyrosine and Tryptophan that shows the structures in the conversions and gives the enzymes
involved) to dopamine, norepinephrine and epinephrine in neurons and in the adrenal medulla gland.

Above: Conversion of phenylalanine and tyrosine to its biologically important derivatives.
Tryptophan
Serotonin
The 5-Sided NH Cyclic Amino Acids: Proline and Histidine
These are characterized by a single 5-sided NH structure as seen below. Their structure relationship to the other alpha-amino acids may not be clear at first but if one considers the C attached to the -COOH like the central C in Glycine, the relationship should be seen.Proline is the only proteinogenic amino acid with a secondary amine, in that the alpha-amino group is attached directly to the side chain, making the α carbon a direct substituent of the side chain. (Note below the wedged bond in the cyclic structure of proline that looks like a long pointer arrowhead away from the +NH2; it means the bond points away from the viewer and into the plane of the paper it is written on)
Schizophrenia and Hyperprolinemia
A metabolic, recessive genetic defect that damages the genes coding for the pyrroline-5-carboxylate dehydrogenase and the proline oxidase enzymes that speed the degradation and excretion of proline and result in its 10 to 15 times elevation of blood test proline is implicated as a cause of schizophrenia. In fact, a recent study has demonstrated mild to moderate increases in blood proline levels in random sampled pre-treatment schizophrenics compared to normal matched control patients.
 |
|
 |
Proline is important in connective tissue such as bone, cartilage and ligament.
Histidine
Note the extra NH2 in Histidine making it strongly alkaline. It forms Histamine in inflammation and allergy and also functions as the alerting neurotransmitter explaining the anti histamine sleep effect.
Amino Acids and Genetic-Code Proteins: The number, linear sequencing and 3-dimensional branching of a protein's Amino Acids (AA) define a protein chemically. A protein may be delineated by its AA combination (alanine-valine-lysine-leucine- …”). Among the 20 alpha AA, the possible structure for protein runs to millions. This will become of great interest when one learns the DNA genetic triplet code.
The Essential Amino Acids: Nine of the 20 AA used for protein synthesis cannot be made from simpler substances. Lack of them in food causes deficiency diseases. They are Methionine for the sulfur AA; Phenylalanine for the 6-carbon ring structure AA; Tryptophan for the complex N-containing ring AA; Histidine for the 5-sided double NH ring AA; and the linear branched AA - Leucine, Isoleucine, Lysine, Threonine and Valine. The others - the non-essential AA - are synthesized in body from glucose, water and other AA (for the nitrogen N atom) and also can be obtained by eating food protein, which is broken down by digestion to supply AA, just like used parts. Essential AA can only be obtained by eating protein that has it or by eating the pure essential AA as pill or powder. The sweetener Aspartame (Nutrasweet) digests to the essential AA phenylalanine and the non-essential AA, aspartic acid. It should not be used by a person with phenylketonuria.
Sources of Protein: Food protein is most concentrated in meat, milk and egg. The white of egg, albumin, is a nonessential-AA protein and the yellow, yolk, is rich in essential sulfur AA. Animal protein has high quality AA but it also has too much saturated fat and too many and too much toxic substances plus additives such as hormone or antibiotic and industrial pollutant. Plant food has a lower concentration of protein but also its protein has less essential AA than animal. Among plants, the mushrooms, nuts and beans have the most protein – in bulk weight and also best in variety of essentials. Plant sources have been processed to produce concentrated protein, as tofu from bean. One defect of a completely vegetarian diet comes from low protein concentration; another, from lack of some essential AA. Also a lack of vitamin B12. A vegetarian, in order to get adequate protein, must eat a lot of food every day for quantity and for variety of AA. Deficiencies of protein and essential AA occur more in vegetarians. Also macrocytic anemias that are treated by B12.The total calories in diet is important in protein nutrition. If you don’t eat enough calories a day to maintain healthy weight, you will start burning protein for energy, which means degrading it to AA and then to fatty acid for energy and it produces body acidosis from liberation of the fatty acids. So, low calories intake (Losing too much weight) results in an increasing requirement for high quality protein.If you have disease that requires specific AA in protein (in protein malnutrition from GI disease), do not try to lose weight. (Similarly if planning major surgery) If you plan to lose weight, be sure you have good food source of high quality protein.
Protein Digestion starts with good food preparation and cooking, good chewing and allowing digestive enzymes to work. Disease or surgery of gut can cause protein malnutrition by diarrhea or poor absorption.
Nitrogen Excretion – Creatinine, Urea and Ammonia: Protein breakdown goes on even with adequate calories. As old cells die, the protein from the old cells can be toxic from high levels of AA released into the blood. So the body has evolved a chemical reaction to break up the AA molecule and excrete the nitrogen part quickly. It is 2-stage.AA --------------->(in liver)------> (Ammonia) NH3 then 2NH3------->(in liver only)------->1 Urea (NH2CONH2). Note that the sign of liver failure requiring transplant is high ammonia in blood and resulting “liver coma.” (Patient smells of ammonia) As one might guess, the liver failure results in very low blood urea. (Opposite of kidneys failure)High-protein diet, because it makes high AA in blood, stresses liver and kidneys. In liver or kidney disease, it is wise to limit protein intake to only essential amino acid protein, also a good idea as preventive health measure against the old age failure of liver and of kidneys. In a patient awaiting liver transplant, life is prolonged by low-protein diet. Similarly with failing kidneys, a renal dialysis may be delayed by low-protein diet. This is a tricky dietary balancing act that needs good nutrition advice because essential AA and calories must be supplied at level to maintain needed protein production but no higher.
Endnote: Low Total Protein (TP) in Blood test is caused by low protein in diet as in starvation. This is of particular importance to persons on a crash weight-loss program of fasting, as I learned on myself. The blood test Total Protein is a good measure of serious malnutrition caused by too much fasting. When it falls below normal during a too prolonged fast or in a famine or a wasting illness, or is lost through the kidney into the urine in the nephrotic syndrome, the blood protein level drops. The very large protein molecules cannot easily pass through the pores in the small blood vessel walls and, being electrically charged, they hold molecules of water inside the blood vessels. When they go low, during rapid weight loss of fasting, the water is released and passes through the pores into the tissues under the skin and we get the dependent edema. It happened to me when my total protein fell below 7,0 grams% and is an infrequently considered cause of dependent edema. One other cause of low total protein blood test is immunoglobulin deficiency, usually due to diseases of white blood cells like leukemia and causing low immunity to infection. If you note low TP, get a protein electrophoresis which will pick up low immunoglobulin.






1 comment:
This is very useful post for me. This will absolutely going to help me in my project. buy steroids
Post a Comment