Physician's Notebooks 2 - http://physiciansnotebook.blogspot.com - See Homepage
2.13c: Whole Body Acid-Base Balance - Update 15 Dec. 2021. The descending list as follows are the important headings the chapter in order as they are met in text
Definition of Acid-Base/Acidity/Akalinity/pH
Acid and Alkali Contribution from Food
Excretion of Acid, the Renal Role and the Special Role of Respiration and Lungs
respiratory alkalosis
respiratory acidosis
Acidosis/Acidemia; Alkalosis/Alkalemia
Metabolic Acidosis
Metabolic Alkalosis
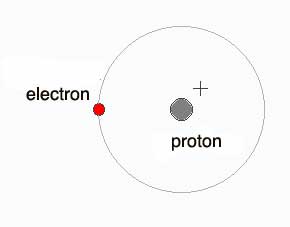
Definition of Acid-Base/Acidity/Alkalinity/pH Acid is any substance that liberates hydrogen ions, H+. If we see an H atom in electron microscopic super vision schematically, it is as below:
In atomic terms, the electron is in an orbital, meaning it does not move like a particle but occupies the whole orbit(al) at any instant; its location can not be pinpointed. Also, note the atom is electrically neutral - the proton has a 1+ and the electron, a 1 - (minus).
A neutral atom tends to lose or gain its electron(s) as its energy input changes. In the case of the H atom, a small input of energy causes it to lose its electron and imbalances it electrically to become 1+ charge due to the unopposed 1+ proton. When a neutral atom becomes charged, it is an Ion (Cation, with + charge; Anion, with minus charge) and, in the case of H, it is written H+, which stands for "hydrogen 1+ charge ion". The concept of acid goes hand in hand with water, the solvent of all life; chemically H2O is more accurately written H.OH to show it comes from the uniting of H+ and OH-. The OH-, which is called hydroxyl radical, is the opposite of acid and typifies bas(e)/(ic), or alkaline. The definition of a base is a substance that liberates OH- or a substance that donates electron(s) to an acid H+. When the acid ion H+ combines with the alkaline ion OH-, the acid and the alkaline neutralize and we get the electronically neutral HOH water molecule.
The importance of acid-base balance in nutrition and health is that the chemical reactions of life are enhanced or inhibited by the acidity or alkalinity of body fluid. Outside of a tightly controlled narrow range of acidity-alkalinity, life is not possible. Acidity and alkalinity should be thought of as a continuum of concentration of H+ in fluid, or written [H+] (the ionic concentration in fluid), with the most acidic fluid having highest [H+] and with the least acidic (most alkaline), having the lowest [H+].
The measure of acidity-alkalinity is pH --- the negative log [H+] to base 10. As pH increases, the [H+] decreases by a power of 10. Example of range of pH from 0 to 14 follows: pH is 0, [H+] is 100, or 1 mEq/L; pH 1, [H+] is 10-1, or 0.1 mEq/L ..., to pH 14, [H+] is 10-14 (Extremely weak H+, strong alkaline solution, exactly it is a decimal of 13 zeros and 1).The pH measurement is done with electric pH probe and is reported accurate to 2 decimal places, i.e., neutral pH is reported 7.00. If we deal with a simple chemical solution in water, in that case, from pH below 7 the fluid is considered acidic, and from pH over 7, it is alkaline. The pH of exactly 7 is neutral with respect to ideal acidity/alkalinity. But in human blood, the plasma pH ranges normally between 7.36 and 7.44, so pH of blood below 7.36 would be considered “acidic” (“acidemia” in blood or “acidosis” in body) and pH over 7.44, “alkaline” (alkalemia, in blood or “alkalosis” in body).Normal pH found in blood plasma is the best value for all the chemical metabolic reactions that make us healthy. So deviation from the normal range means disease. The pH recorded from blood represents the sum of acid and base in food and drink absorbed from your GI tract (includes medications) plus the acid and base produced and dissipated by body metabolism and excreted by kidneys in urine, by lungs in exhaled air, and from GI tract in secreted and lost stomach and intestinal fluids. Since acidity/alkalinity of the food we eat varies, day by day, the mechanism by which we are able to keep blood pH in healthy range is mostly by acutely excreting more or less acid as ammonium ion (NH4+) in urine and also breathing away more (decreasing acidity) or less (increasing acidity) CO2 from lungs. If we eat too much acid-producing food on a particular day, the kidneys compensate by excreting extra acid in the form of NH4+ (NH3 ammonia plus H+) in urine to balance it and, the blood (and body fluid) pH remains unchanged.Acidity or alkalinity of blood may be disturbed by poisoning: for example, ethyl alcohol is a strong acid-producer so if you get drunk, your blood pH will drop to acidemia state (<7.32).Also, certain diseases produce acid: Diabetes mellitus in absence of enough insulin produces keto-acids from breakdown of flesh that releases fatty acids leading to diabetic acidosis and low pH. Poor kidney function results in inability to excrete acid in urine so persons in kidney failure get severe acidosis with low body fluid pH. Severe illness or lack of oxygen produce a low pH due to lactic acid production by low-oxygen tissues.
Acid and Alkali Contribution from Food: The American diet contributes more acid than alkali. Important acid-producing foods are the sulfur-containing protein amino acids found in egg yolk and meat. Protein metabolism also produces uric acid as end product. Alkali-producing food contains much sodium, potassium and calcium as cations (Positive charges). The organic anions (negative charge ions) that they combine with, proteinate, and citrate, are mostly metabolized and excreted from lungs as CO2, leaving the alkali Na+ cation unopposed. These cations are found in milk and its products, and in fruit and vegetables. (Yes! Fruit although it may have immediate acidic effect in stomach, contributes to body alkalinity after it is metabolized!) In special situation (i.e., kidneys failure, diabetes) it is important to know whether a food may have acid or alkaline effect on body fluid.
Excretion of Acid, the Renal Role and the Special Role of Respiration and Lungs: by CO2-HCO3- in KeepingNormal Blood pH – Urine is a main route whereby your body rids itself of acid by using ammonia (NH3) like a magnet to pick up excess H+ from blood that flows by the excretory cells in the kidneys, and then urinates out the combination (NH3 + H+) as the ammonium ion NH4+. In acidosis, urine, when exposed to air, immediately smells strongly of ammonia. (On long-time standing all urine will smell of ammonia)
The ammonia excretion mechanism is combined with the CO2 -HCO3- acid/base balance mechanism involving CO2, the end product of carbohydrate and fat metabolism in the body. Carbon dioxide (CO2) excretion in lungs is under mostly voluntary control since it depends on the rate and depth of breathing, which has a wide range for bringing oxygen O2 into your body and getting rid of the CO2. Test yourself and see! You can breathe at a rate that starts from as low as 6 breaths a minute and goes as high as 40 and can get enough oxygen to sustain life and activities; but there is great difference in blood [CO2] excretion between respiratory rates of 6 and 40 at the same depth breaths per minute. The faster you breathe at the same depth of each breath the more CO2 you excrete in unit time and the lower becomes your blood [CO2]. And, of course, you can vary the depth of respiration. So the breathing rate and depth is a way to gain or lose CO2. And this goes beyond voluntary removal since a sensor in the base of your brain is sensitive to the [CO2] in arterial blood that flows by it. When arterial [CO2] is low, this sensor automatically slows down the involuntary part of your breathing rate (like when asleep) and, when arterial [CO2] is high, the rate is increased.
The CO2 in blood plasma reacts, inside cells, with water to form hydrogen carbonate, (H2CO3, more commonly known as carbonic acid, in fizzing soda). The chemical reaction is: CO2 + H2O ーー>H2CO3Hydrogen carbonate is a weak acid but in kidney cells the coupled chemical reactions serve the function of excreting H+ in urine as NH4+ and of releasing HCO3- into the blood, which, combines with an H+ in lungs and breaks down to H2O (water which is neutralized H+) and CO2 (carbon dioxide gas) that is exhaled from the lungs.So CO2 being exited from your body by blowing it out in exhalation breaths in the lungs is an important, added mechanism for self protection against excess acidity, because it ties up the excess H+ by putting it into the acid-base neutral water (HOH) molecule and blows away the CO2. (i.e., if there is acidosis - low pH, high [H+] -, the excess H+ is tied up by HCO3- made in your kidneys, and the resulting H2CO3 splits into neutral H2O and CO2, and the increased rate of breathing excretes the CO2, which pulls the reaction to neutralize more H+ and restore blood pH to normal) So in addition to the urine NH4+ mechanism, the lung CO2 exhalation protects against acidosis caused by disease or diet.Sometimes, nervousness starts one breathing fast or deep, (hyperventilation). In one who starts with normal blood pH, hyperventilation, by blowing off CO2, lowers [H+] too much, raises pH and causes alkalosis. This is called “respiratory alkalosis” because it occurs from increased breathing rate or depth. In addition to nervous hyperventilation, certain medicines in overdose cause hyperventilation resulting in respiratory alkalosis. Most famous is aspirin.The opposite of respiratory alkalosis is respiratory acidosis where the reverse happens. One breathes too slowly and/or not deeply enough or disease in lungs causes block in CO2 exhalation from lungs. Result is that the CO2 loss falls behind its production and, in blood, the [CO2] rises, leading to increasing [H+] and lowering of pH to acidosis. In response, the kidneys make excess HCO3– to neutralize the excess H+ giving reactive alkalosis and a high [HCO3-] (higher than 25 mEq/L). Thus, the breathing rate and the kidneys combine to compensate for acidosis in blood.
Acidosis/Acidemia; Alkalosis/Alkalemia: Acidosis (body condition of low pH) also “acidemia” (pH of blood <7.36) and alkalosis or alkalemia when the pH is >7.44 are exact definitions but practically as derangement and symptomatic disease, a severe acidosis means pH < 7.20, and a severe alkalosis, >7.60.The blood pH is a fundamental measure of your health status, like basal body temperature. Its derangement means something has gone seriously wrong, either metabolic (due to derangement of body metabolism and usually involving kidneys) or respiratory (due to lungs and respiration). We have already dealt with respiratory alkalosis and acidosis so now let’s deal with metabolic counterparts.
Metabolic Acidosis Showing Low [HCO3-] less than 20 mEq/L with acidemia, plasma PH (<7.36)
One type has normal kidney function. It is due to abnormal acid in plasma, as in diabetic acidosis where acids flood blood, or it can happen during and after an alcoholic drunk when acid products of ethyl alcohol do it; in both cases overwhelming the kidneys’ ability to neutralize excess [H+].
Metabolic Acidosis from Kidney Problem: In kidney failure, acidosis is part of end stage that needs kidney dialysis. Blood tests show BUN (Blood Urea Nitrogen) more than 25 mg% and creatinine more than 1.5 mg% and plasma pH is less than 7.36, the typical metabolic acidosis due to the failing kidneys' inability to excrete sufficient H+ to balance the H+ produced in metabolism or in the diet.Practical point in trying to delay or avoid kidney dialysis is to reduce intake of acid-forming foods. Most obvious is food that contains protein with sulfur amino acid like yellow of egg and red meats. Food and drink that leaves alkali residue (vegetable & citrus fruit) should be favored. In some case sodium bicarbonate is given but its Na+ may be problematic.
Metabolic Alkalosis shows blood pH higher than 7.44 and [HCO3-] higher than 25. Usually the alkalosis is due to loss of acid as in prolonged vomiting or taking too much bicarbonate antacid for stomach problem. Also it could reactive, as in a heroin addict whose breathing rate and depth is decreased, causing respiratory acidosis and the kidney over reacts to make a compensatory metabolic alkalosis which needs no treatment.Prevention and treatment of metabolic alkalosis, when needed, is to stop loss and treat vomiting dehydration (if due to those factors) not with pure water only but with mixed electrolyte fluid as is found in sports drink. And stop antacids! Antacids are medications that neutralize acid in stomach.Chapter 13 continues section 13d on acid/base and diseases. Click 2.13d Alkaline & Acid Tides of Body/Respiratory C...
No comments:
Post a Comment